Metals and non-metals in the Periodic Table
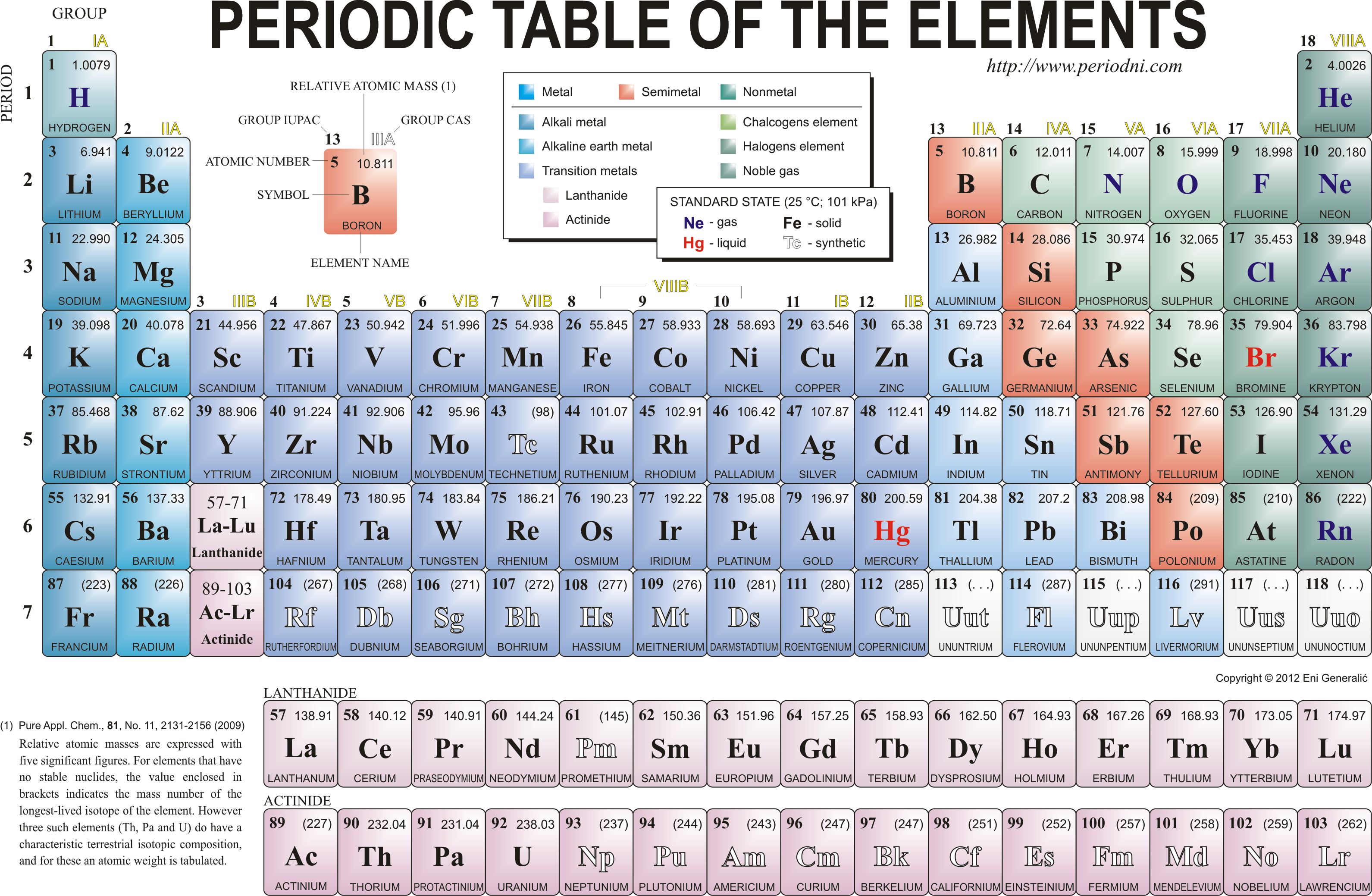
From left to right across a period there is a gradual change from metal to non-metal. For example, in Period 3, sodium, magnesium and aluminium are metals. They all conduct electricity and their oxides are basic. Phosphorus, sulfur, chlorine and argon are non-metals, They are all poor conductors of electricity and the oxides of P, S and Cl are acidic. Silicon has some properties of both a non-metal and a metal, so is therefore called a semi-metal or (metalloid). Silicon is a semi-conductor of electricity and is used in computer chips for this reason. Silicon dioxide is acidic.
No comments:
Post a Comment